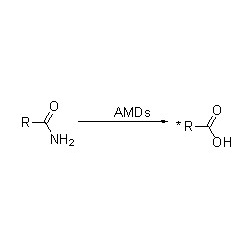
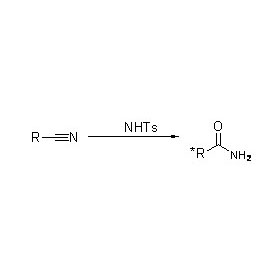
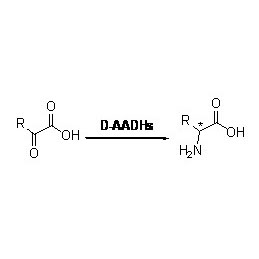
Amidase (AMD)
Enzymes:Ko macromolecular biological catalysts, ma enzymes mazhinji mapuroteni
Amidase:Catalyze iyo hydrolysis yeakasiyana endogenous uye ekunze aliphatic uye anonhuwirira amides nekuendesa acyl boka kumvura nekugadzirwa kwemahara acids uye ammonia.Hydroxamic acids uye mamwe ma organic acids anoshandiswa zvakanyanya semishonga nekuti iwo anoumba ekukura zvinhu, mishonga inorwisa mabhakitiriya uye tumor inhibitors.Iyo amidases inogona kukamurwa kuita R mhando uye S mhando acylases zvinoenderana neiyo catalyst stereoselectivity.
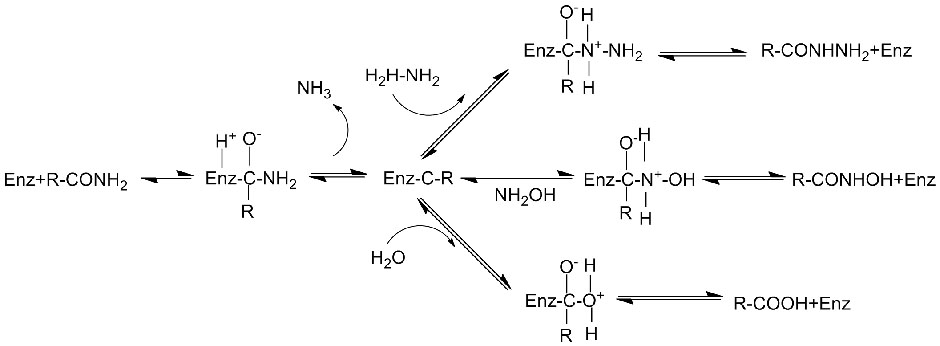
Pamusoro pekukonzeresa iyo hydrolysis yeamides, amidase inogona zvakare kukonzeresa maitiro ekutamisa acyl pamberi pe-co-substrates senge hydroxylamine.
Amidase ine akasiyana masosi ane akasiyana substrate chaiyo, mamwe acho anogona chete hydrolyze anonhuwirira amides, mamwe acho anogona chete hydrolyze aliphatic amides, uye mamwe hydrolyze α-kana ω-amino amides.Mazhinji emaamine ane yakanaka catalytic chiitiko chete kune acyclic kana nyore anonhuwirira amides, asi kune yakaoma aromatics, heterocyclic amides, kunyanya amides ane ortho substituents, anowanzo akaderera mukuita (ma enzymes mashoma anoratidza zvirinani zvinokonzeresa mhedzisiro).
Catalytic mechanism:
| Enzymes | Product Code | Product Code |
| Enzyme Powder | ES-AMD-101~ ES-AMD-119 | seti ye19 amidases, 50 mg imwe neimwe 19 zvinhu * 50mg / chinhu, kana humwe huwandu |
| Screening Kit (SynKit) | ES-AMD-1900 | seti ye19 amidases, 1 mg imwe neimwe 19 zvinhu * 1mg / chinhu |
★ High substrate chaiyo.
★ Strong chiral selectivity.
★ High kutendeuka kushanda zvakanaka.
★ Zvishoma ne-zvigadzirwa.
★ Mild reaction conditions.
★ Kuchengetedza zvakatipoteredza.
➢ Kutariswa kwe enzyme kunofanirwa kuitirwa kune chaiwo substrate nekuda kweiyo substrate chaiyo, uye kuwana enzyme inokonzeresa iyo inotarirwa substrate ine yakanakisa catalytic mhedzisiro.
➢ Usambofa wakabata nemamiriro akanyanya akadai se: tembiricha yepamusoro, yakakwirira/yakaderera pH uye organic solvent ine yakanyanya kugadzikana.
➢ Kazhinji, iyo reaction system inofanirwa kusanganisira substrate, buffer solution (Iyo yakanyanya kuita pH ye enzyme).Co-substrates senge hydroxylamine inofanira kuvapo mu acyl transfer reaction system.
➢ AMD inofanirwa kuwedzerwa kwekupedzisira mukuita system ine optimum reaction pH uye tembiricha.
➢ Ese marudzi e AMD ane akasiyana optimum maitiro ekuita, saka imwe neimwe yadzo inofanirwa kuenderera mberi ichidzidzwa yega.
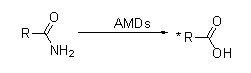
Muenzaniso 1(1):
Hydrolysis chiitiko kune akasiyana Amide Substrates
| Substrate | Chiitiko chakananga μmols min-1mg-1 | Substrate | Chiitiko chakananga μmols min-1mg-1 |
| Acetamide | 3.8 | ο-OH benzamide | 1.4 |
| Propionamide | 3.9 | p-OH benzamide | 1.2 |
| Lactamide | 12.8 | ο-NH2benzamide | 1.0 |
| Butyramide | 11.9 | p-NH2benzamide | 0.8 |
| Isobutyramide | 26.2 | ο-Toluamide | 0.3 |
| Pentanamide | 22.0 | p-Toluamide | 8.1 |
| Hexanamide | 6.4 | Nicotinamide | 1.7 |
| Cyclohexanamide | 19.5 | Isonicotinamide | 1.8 |
| Acrylamide | 10.2 | Picolinamide | 2.1 |
| Metacrylamide | 3.5 | 3-Phenylpropionamide | 7.6 |
| Prolinamide | 3.4 | Indol-3-acetamide | 1.9 |
| Benzamide | 6.8 |
Maitiro akaitwa mu50mM sodium phosphate buffer solution, pH 7.5, pa70 ℃.
| Amides | Hydroxylamine | Hydrazine |
| Acetamide | 8.4 | 1.4 |
| Propionamide | 18.4 | 3.0 |
| Isobutyramide | 25.0 | 22.7 |
| Benzamide | 9.2 | 6.1 |
Maitiro akaitwa mu50mM sodium phosphate buffer solution, pH 7.5, pa70 ℃.
Related reagent concentration: amides, 100 mM(benzamide, 10 mM);hydroxylamine uye hydrazine, 400 mM;enzyme 0.9 μM.
Muenzaniso 2(2):
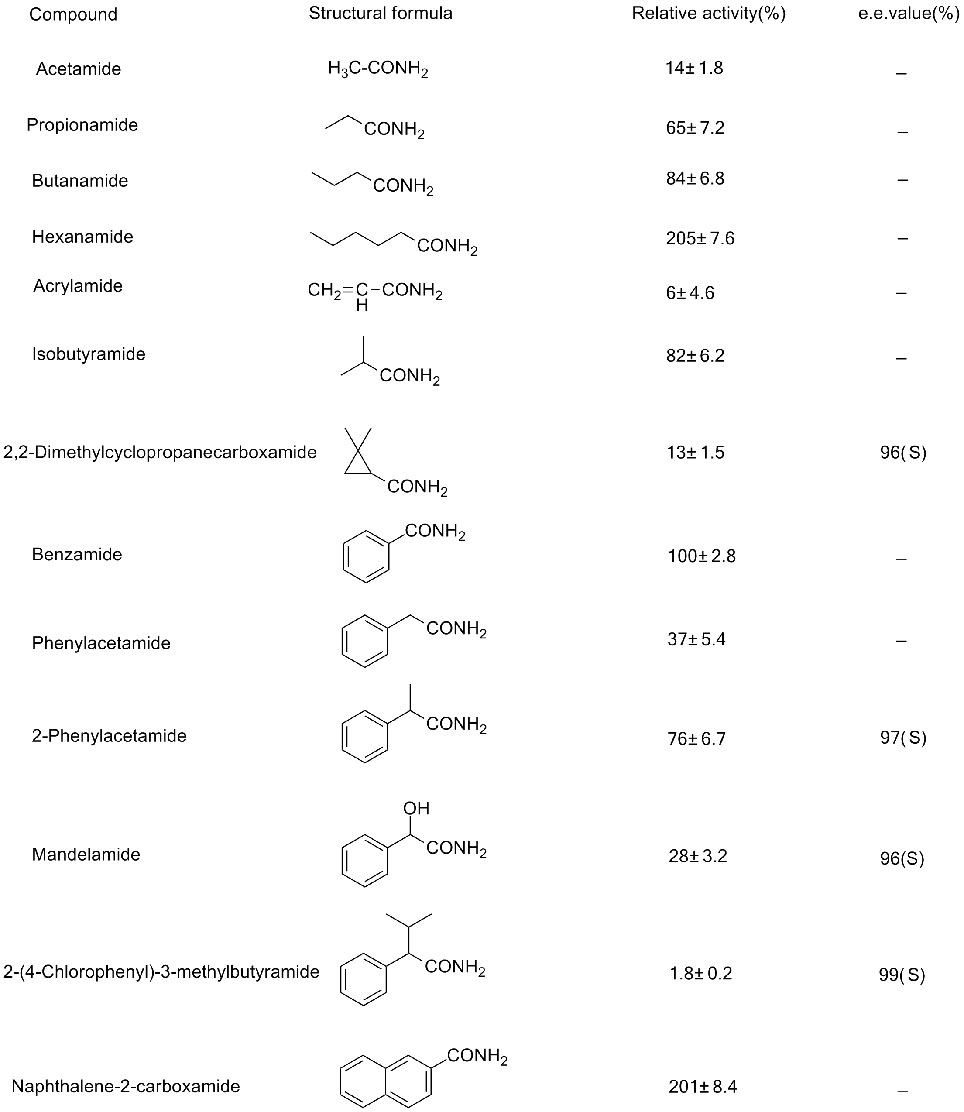
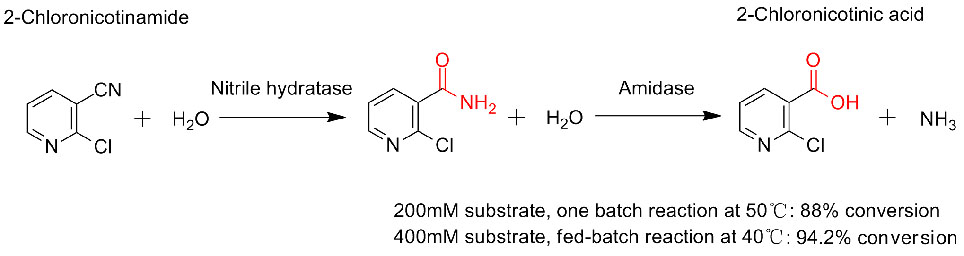
Muenzaniso 3(3):
1. D'Abusco AS, Ammendola S., nevamwe.Extremophiles, 2001, 5:183-192.
2. Guo FM, Wu JP, Yang LR, nevamwe.Process Biochemistry, 2015, 50 (8): 1400-1404.
3. Zheng RC, Jin JQ, Wu ZM, et al.Bioorganic Chemistry, 2017, Inowanikwa online 7.